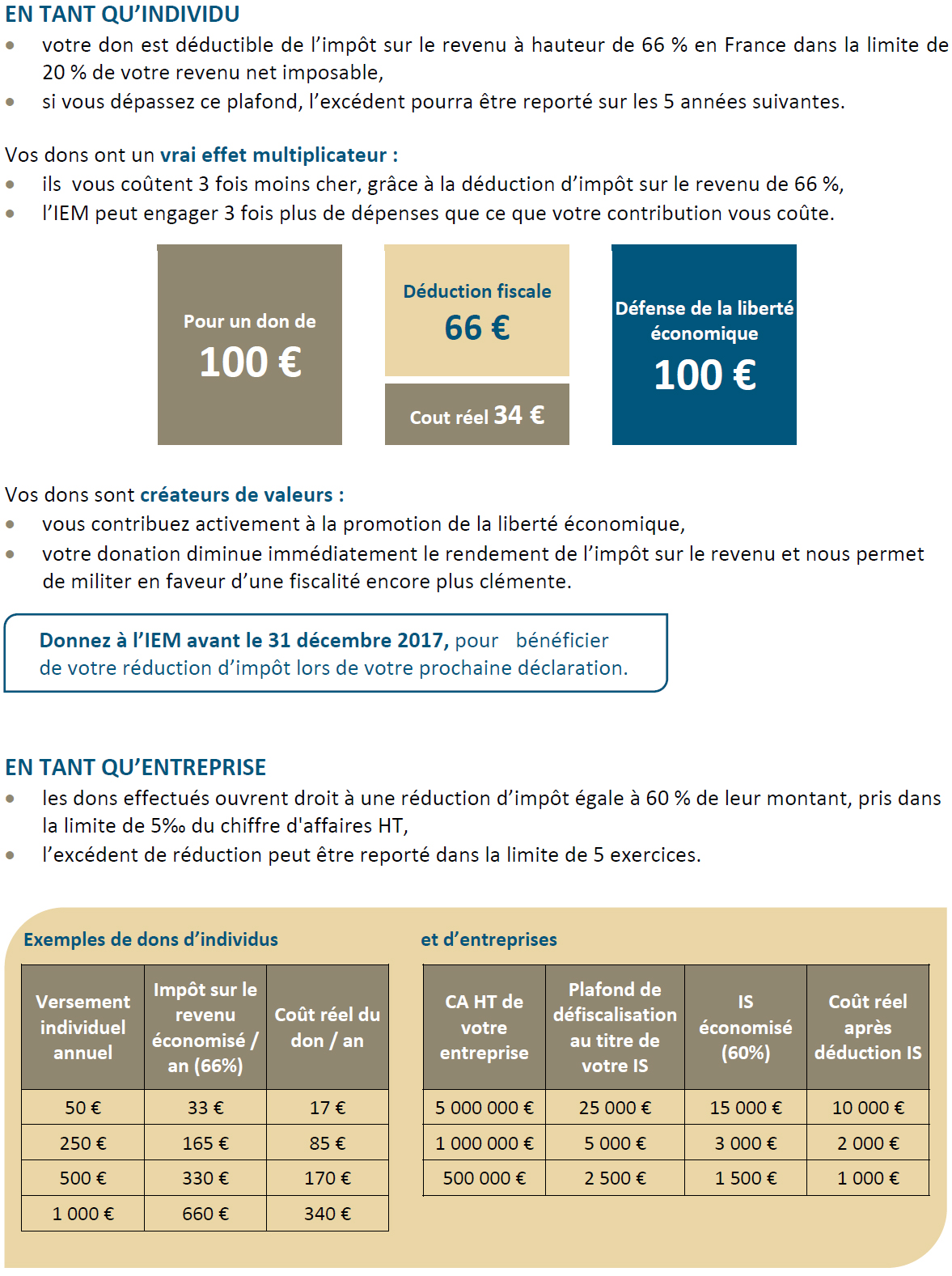
Therapeutic Substitution Policy – A Further Step in the Bureaucratisation of Drug Use
Article published in the EU Reporter Magazine (nov. 2007-jan. 2008, p. 22).
Although prescription, use and reimbursement of drugs policies can reduce some pharmaceutical spending by favouring systematically the use of cheaper medicines, this involves a bureaucratisation of drug use that presents risks for the health of the insured and a potential increase in other health care or patients’ costs.
With the aim of controlling health care spending, public authorities in several countries increasingly are regulating the prescription, use and reimbursement of drugs. One of the latest examples is therapeutic substitution policies concerning drug therapies. Although such policies can reduce some pharmaceutical spending by favouring systematically the use of cheaper medicines, this involves a bureaucratisation of drug use that presents risks for the health of the insured and a potential increase in other health care or patients’ costs.
Therapeutic substitution policies have already been implemented in various forms not only in Europe – in countries such as Germany or the United Kingdom – but also for example in New Zealand or Canada. Their principle is simple: facing the high cost of certain products, public authorities put doctors and patients under pressure to replace them with cheaper, brand name or generic (but not bio equivalent), therapies.
Accordingly, public authorities put certain drugs together in the same category because they supposedly treat the same conditions, even if their chemical composition is different, their effectiveness weaker or their side effects stronger.
But therapeutic substitution also relies on highly specific coverage policies that cause patients to change therapy. For instance, the health insurance system may decide to cover drugs in the same category less if they are priced higher than another drug chosen as a reference – often among the least expensive in the category (reference pricing system). Insured persons who must pay on their own for a more expensive drug – as is the case in countries including Germany or New Zealand – have a powerful incentive to change to a less expensive but more broadly covered therapy.
Health insurance systems, as in British Columbia (Canada), may also decide to cover only one drug – among the less expensive – in a given class. The incentive for patients to switch is then even stronger: other than the drug in question, no drugs at all are covered!
Therapeutic substitution policy may also be implemented by giving financial incentives to doctors – as in the United Kingdom since 2004 – to change therapies on a broad scale, abandoning some high-cost therapy for another different, but less expensive, therapy.
However, this sort of substitution by cheaper drugs, sometimes conducted on a broad scale for all insured persons, has its own costs and risks, even if it may create savings in the budgetary item of some specific pharmaceutical product.
A change in therapy could result in the administration of a less effective drug with more side effects and different interactions with other drugs taken by patients. For example, people covered under the public plan in New Zealand, where this type of policy was instituted in the late 1990s, were pressured into substituting a less expensive drug for their anti-cholesterol therapy. The drug in question was judged by bureaucrats to be perfectly substitutable, but it turned out that those who switched experienced considerable rises in cholesterol levels and were thus at higher risk of cardio-vascular incidents.
In the Canadian province of British Columbia, six months after the establishment of a therapeutic substitution policy for anti-ulcer drugs in July 2003, nearly 25% of patients who chose the less expensive referenced drug had to stop taking it because of its ineffectiveness or major side effects.
Closer to home, there is the example of the United Kingdom. The risks from the change between two anti-cholesterol drugs were observed in a study conducted by a cardiology specialist at the North Staffordshire University Hospital. The drug switching from atorvastatin to simvastatin was associated with a mortality rate more than three times higher as well as increased patient readmission rates to hospital following cardiac events. Another study on switching between the same drugs by the pharmaceutical company Pfizer and recently presented at the annual conference of European cardiologists in September 2007 in Vienna also leads to suspect the existence of similar health risks.
Complications following a change in therapy can end up requiring more visits to a doctor or a hospital. Even if a public system can reduce some pharmaceutical spending, bureaucratisation in drug use can thus also cause an increase in other areas of health care spending as well as non-monetary but very real costs for patients in terms of lost time or greater suffering.
Seeking to present some cheaper drugs as substitutable for patients could indeed be in itself a legitimate strategy for an insurance provider. However, for such a policy to be truly beneficial to patients and for its risks to be taken effectively into account, it must be subject to competition and to the « market test ».
Insured persons, acting under their doctors’ advice, should have the option of changing health insurance plans if they feel that therapeutic substitution is not merely inadequate and lacking in added value in their eyes but that, on the contrary, it increases the risks to their health illegitimately. It shouldn’t be forgotten that this, unfortunately, is not the case with our compulsory health insurance systems.
Valentin Petkantchin, Research Director, Institut économique Molinari