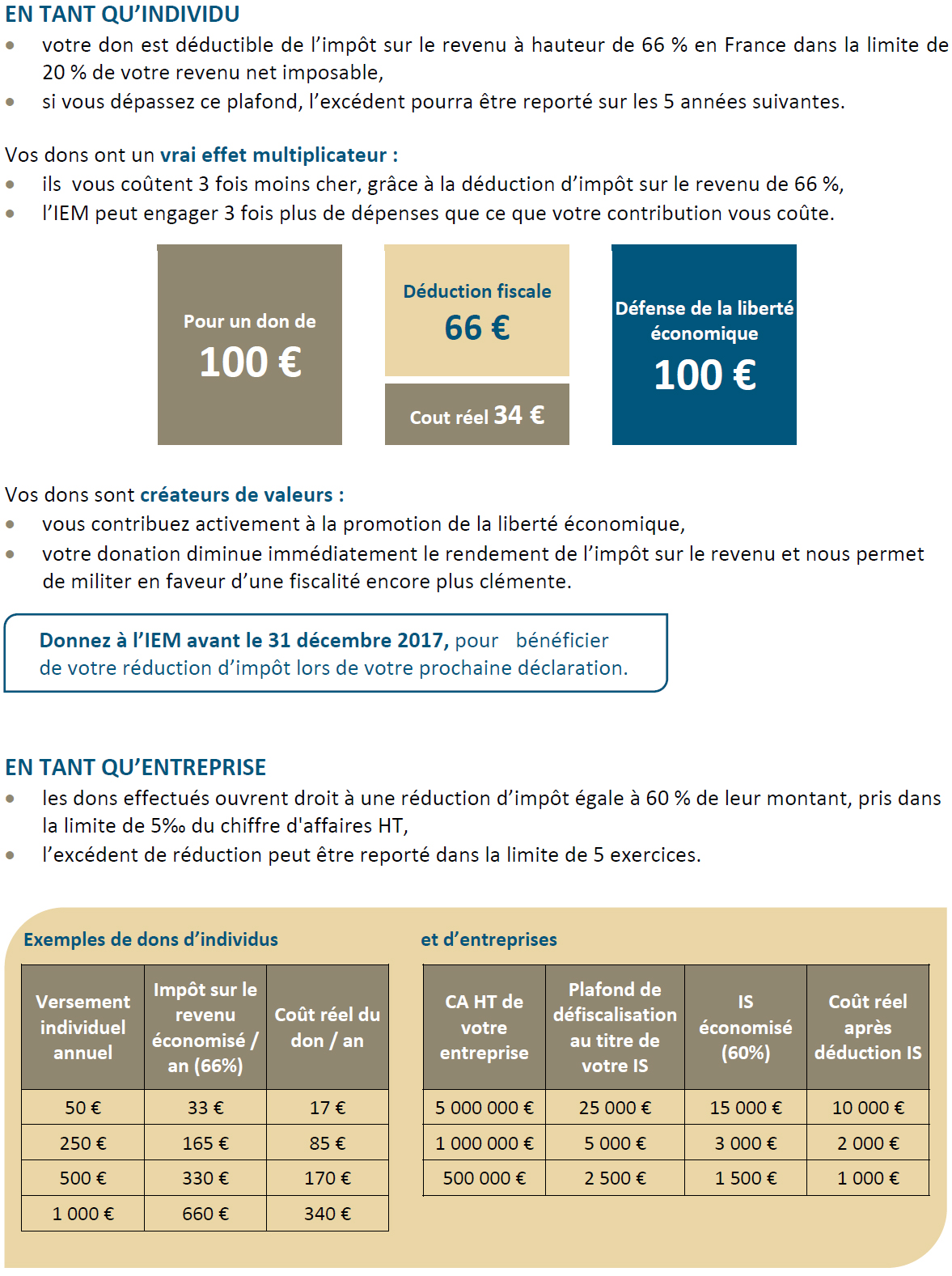
Advertising restriction represents a health hazard
Article published in the Financial Times on January 10, 2006.
Patients want more information. With ageing populations and higher standards of living, patients’ concern for their future drives a growing supply of information in various forms. Television programmes, books and newspapers and the internet focusing on health are proliferating at terrific speed. But the advertising of prescription drugs is banned across the European Union through a directive dating from 1992 that restricts such pluralism in the supply of information.
Some individual EU member states are even more tightly regulated. In France, any information supplied direct to the public by manufacturers is considered to be advertising, while Italy bans the advertising of prescribed medicines. Hence, health consumers depend predominantly on their family doctors for information concerning new therapies and products.
Although it is generally agreed that freedom of information is beneficial to consumers, pharmaceutical advertising is considered too dangerous to be handled by the primary constituency: health consumers. Nevertheless, recent surveys in EU countries, such as the Populus/Stockholm Network survey of 2004, show that patients increasingly want more influence over their treatment and drugs. Patients may care deeply about equality of access, but healthcare systems are finding it hard to cope with the consumer approach to services that have so far been managed through waiting lists and the shifting priorities of political expediency. The situation has become more complex with recent European Court of Justice rulings that EU member states must reimburse patients for treatment in other countries if their national health service is unable to meet demand.
In order to be interested in any product, consumers need first to be aware that such a thing is available. This is why entrepreneurs and companies invest in communication and marketing. Companies like advertising because it alerts consumers to their products, but this does not mean it is not also providing information. Entrepreneurs are well aware information is an essential factor of production.
However, there are many objections when prescription drugs are involved. A common claim is: “Drugs are not like other products.” In fact, drugs are exactly like other products to the extent that they are produced in order to fulfil a need. It is also said that “drugs are dangerous”. True, but so are any number of products that are handled without proper information and advice. Automobiles are highly dangerous and expensive. Hence, purchasing a new car involves consumer cost-benefit analysis, including of safety features. Indeed, this is literally an issue of life and death. Advertising is one important source of information among others, but that does not mean consumers will buy a new car only because they have seen an advertisement.
Another objection is that “advertising downplays safety”. In justification of strict government regulations, producers are regularly accused of stressing the benefits and minimising the risks associated with their products. But an important difference is that companies suffer immediate sanctions through financial loss if they neglect their consumers, unlike bureaucrats whose income does not depend directly on their services. In addition, this view assumes that companies are the sole providers of information, whereas pluralism demands a much wider array of suppliers. The fact that publishers rely on advertising does not imply that books are getting positive reviews.
It would be no more useful in terms of consumer information to condemn advertising in principle than it would be to cite possible deficiencies of campaign materials as evidence of manipulation of voters in democratic elections. Only a competitive market for health consumer information (including patient and consumer organisations and companies) can improve choice for individuals.
Advertising practices for prescription drugs, where authorised, certainly leave room for improvement, but this is more a question of reviewing regulations to make more information available to consumers than of tightening restrictions. The basic issue remains the free access to information, commercial or otherwise, for the benefit of health consumers as sovereign decision-makers. In this context, the EU ban on advertising is a big obstacle to the quest for more and better information on medicines.
Jacob Arfwedson is associate researcher at the Institut Economique Molinari (Brussels) and Alberto Mingardi is executive director of the Istituto Bruno Leoni (Milan)